Product Information
Product Name | Product Number | Specification | store |
HRbio™ Zero TOPO-TA/Blunt Cloning Kit, MCS-Depleted Zero background TOPO-TA/Blunt Universal TOPO cloning kit, without MCS multiple cloning restriction site | HRF0161 | 20T | -20℃ |
HRbio™ Zero TOPO-TA/Blunt Cloning Kit, MCS-Depleted Zero background TOPO-TA/Blunt Universal TOPO cloning kit, without MCS multiple cloning restriction site | HRF0162 | 80T | -20℃ |
Product Description
This product is different from the traditional T4 ligase principle. It uses the principle that Topoisomerase can connect DNA fragments instantly (a few seconds to a few minutes) and efficiently (close to 100%), and is made using our company's original process.
1. Any PCR product (compatible with A-end/blunt-end) can be connected in an instant (seconds to minutes).
2. The size of the specially designed new vector plasmid is less than 2kb, which gives full play to the advantage that the smaller the TOPO vector is, the larger the fragment it can accommodate, and maximizes the efficiency of large fragment connection; the size of the plasmid after connection is more than 2kb smaller than the traditional vector. The smaller the plasmid, the higher the transformation efficiency, which greatly increases the number of transformants after the connection of various fragments.
3. The recovery time of ampicillin-resistant vector is only 10 minutes, which is 6 times shorter than the 1-hour recovery time of kanamycin-resistant vector.
4. The fastest way to complete the transformation is to use room temperature within 5 minutes without ice bath and heat shock ...
5. Zero background principle of suicide gene, no false positives, no need for tedious blue-white screening and colony PCR screening. In most cases, a randomly selected clone will have an insertion (close to 100%).
6. The ability to connect long fragments far exceeds that of traditional TA/Blunt cloning vectors, and can connect fragments up to 10kb (for example, by connecting a 5kb fragment, it is possible to pick 10 colonies, at least 8 of which have insertions). It is a new generation of simple, fast, zero-background, screening-free TOPO TA/Blunt cloning vector.
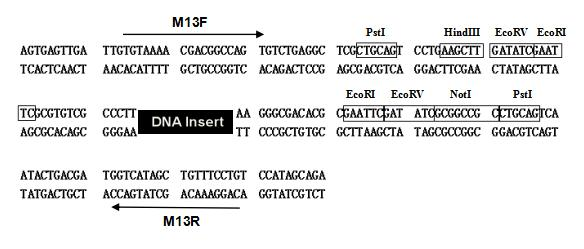
Note: Sequencing can only be performed using the M13F/M13R universal primers (see the following diagram), but not the M13(-47)/M13(-48) universal primers.
The same primers as those for sequencing can be used for PCR.
Product Usage
Cloning of PCR products.
Rapid sequencing of PCR products after cloning (using M13F/M13R primers).
Product composition
Component No. | Component name | Product number (specification) | |
HRF0161(20T) | HRF0162(80T) | ||
HRF016-A | TOPO-TA/Blunt Simple Vector(30ng/μL) | 40 μL | 160 μL |
HRF016-B | 1000bp Control (30ng/μL) | 5 μL | 5 μL |
HRF016-C | 10× Enhancer | 20 μL | 80 μL |
Transportation and storage methods
Transported with dry ice. Store at -20℃ to avoid repeated freezing and thawing. Valid for one year.
Precautions
1) Sequencing can only be performed using the M13F/M13R universal primers (see the following diagram), but not the M13(-47)/M13(-48) universal primers.
The same primers as for sequencing can be used for PCR.
2) For your safety and health, please wear a lab coat and disposable gloves when operating.
3) This product is for scientific research purposes only!
How to use
1. Preparation for ligation reaction:
PCR primers can be used with normal design without any changes (phosphorylated primers cannot be used). Any PCR product (compatible with A-terminal/blunt-terminal) can be directly connected. It is generally recommended to recover and purify the PCR product by gel to avoid possible problems later. If the PCR product only has the target band, no non-specific bands and primer dimers, you can also try to directly connect the reaction. If the PCR product is based on a plasmid as a template, it is best to purify it, because the template plasmid may also grow colonies (but not the target vector you want to construct).
2. Ligation reaction:
1) Set up a 10μL ligation system at room temperature (25℃-37℃) (0.2ml PCR tubes and PCR instrument temperature control are recommended):
| Purified PCR product/or 1μL 1000bp control | 0.5-5μL |
| TOPO-TA/Blunt Vector | 2μL |
| 10 x Enhancer | 1μL |
| Sterilized water | XμL |
| Total volume | 10μL |
After adding the reagents, use a pipette to gently blow and mix or tap the bottom of the tube to mix. Centrifuge at low speed to collect all the liquid at the bottom of the centrifuge tube .
The steps cannot be performed on ice and must be performed at room temperature (25°C-37°C).
Note: If you use a 5μL system for ligation, use half of each component in proportion and double the number of uses.
Amount (note that too much will lead to a decrease in transformants):
| Insert size (bp) | Recommended dosage (ng) |
| 100-1000 | 10-40 |
| 1000-2000 | 40-80 |
| 2000-5000 | 80-180 |
2) Ligation at room temperature (25℃-37℃) for 5 minutes. This vector is recommended to complete the ligation at room temperature (25℃-37℃) for 5 minutes.
For difficult fragments, the ligation time can be extended to 10-15 minutes, and the temperature can be selected to be 37°C, which can significantly increase the number of transformants.
3) Place the ligation product on ice until ready for use. Immediately proceed to the standard competent transformation procedure or the rapid transformation procedure.
3. Fast conversion:
1) Take out the competent cells from -80℃ and quickly put them into an ice bath to thaw (about 1-3 minutes).
2) Immediately add 5 μL of ligation solution (you can add all of it as long as the volume does not exceed 1/10 of the volume of competent cells), and shake the bottom of the centrifuge tube by hand.
Mix gently (avoid pipetting) and place on ice for 5 minutes.
3) Heat shock in a 42℃ water bath for 60 seconds, then quickly return to the ice bath and let stand for 2-3 minutes. Do not shake the centrifuge tube during this process.
Notice:
For this step, it is recommended to heat shock in a 42℃ water bath for 60 seconds. However, according to our experience, most commercial TOP10 and DH5a competent cells can also be placed in the centrifuge tube at room temperature (˃22℃) for this step. The time does not need to be very accurate. In summer or when the room temperature is high, it can be placed for about 5-8 minutes; if the room temperature is low, the time can be extended to about 8-15 minutes. Experienced customers can try non-heat shock transformation according to specific circumstances.
4) Add 300-500μL LB or SOC medium (without antibiotics) and shake culture at 37℃ 200 rpm for 10 minutes. According to our experience, the culture medium (if it is taken out of the refrigerator and the temperature is low, it should be placed in a 37℃ incubator to warm up to 22℃-37℃ in advance) can be directly added to the 1.5 ml centrifuge tube of competent cells, covered with the centrifuge tube cap, fixed horizontally in a shaking incubator for shaking culture and recovery, and no need to transfer to a test tube for culture and recovery. Generally, commercial competent cells do not exceed 2kb insert fragments. After heat shock, 10-15 minutes of recovery can obtain enough transformants. If the efficiency of laboratory-made competent cells is low, or there are few transformants and the insert fragment is long, the recovery time can be increased to 30-60 minutes to obtain more transformants.
5) Take 100-200μL of bacterial solution and apply it to the plate (the culture plate contains 100μg/ml ampicillin), and culture overnight. (If the number of transformants is expected to be small, in order to obtain more clones, centrifuge at 4000 rpm for 1 min, discard part of the supernatant, retain 100-150μL, flick the suspended bacteria, and apply all the bacterial solution to the plate)
4. Screening and identification of transformants:
This product uses the suicide gene zero background principle. Bacteria without insertion will commit suicide and cannot grow, so there are almost no false positives. Generally, what you see is what you get. As long as the colonies grown are normal (not contaminated bacteria, and the number of transformants is not too small), they basically contain insertions. Therefore, if the inserted fragment does not exceed 2-3kb, colony PCR identification is not required, and 1-2 bacteria can be directly selected for sequencing.
1). Our company's TOPO vector has a very high positive rate, so if the colony growth is normal and the number is not too small, it is recommended to skip the colony PCR/bacterial liquid PCR identification and go directly to sequencing. Note that the sequencing primer cannot use M13 (-47)/M13 (-48) universal primer sequencing.
2). The colony PCR results of TOPO vectors are prone to false negatives. Therefore, when using colony PCR for identification, if the colony PCR result is positive, this result can generally be trusted. If the result is negative, or the amplified size does not match the expected size, it is generally not trusted, and the possibility of false negative colony PCR results should be considered. It is necessary to further extract the plasmid electrophoresis size or enzyme digestion identification to confirm.
3). Use the bacterial liquid of the white colonies cultured above to extract the plasmid. If the inserted fragment is large, you can directly identify the inserted plasmid by checking the plasmid size by running electrophoresis. You can also use EcoR I/Ecor V single enzyme digestion to release the inserted fragment or use other appropriate enzyme digestion, and check the fragment size by agarose gel electrophoresis to determine whether it contains the target fragment.
TOPO-TA/Blunt carrier map:

TOPO-TA/Blunt vector
universal M13 sequencing primer sequence:
M13F:TGTAAAACGACGGCCAGT
M13R: CAGGAAACAGCTATGACC
Note: "M13 universal primer" has a variety of different sequences, and the default M13 primer of some primer synthesis companies is different from the M13
sequence . Be sure to check the sequence before synthesis.
TOPO-TA/Blunt vector multiple cloning site sequence: