Product Information
Product Name | Product Number | Specification |
Dual Luciferase Reporter Gene Assay Kit | HRK1208 | 100 times |
Dual Luciferase Reporter Gene Assay Kit | HRK1209 | 1000 times |
Product Description
Firefly luciferase is a protein with a molecular weight of about 61 kDa . In the presence of ATP , magnesium ions and oxygen, it can catalyze the oxidation of luciferin to oxyluciferin , and emit bioluminescence with a wavelength of about 560 nm during the oxidation process. Renilla luciferase is a protein with a molecular weight of about 36 kDa . In the presence of oxygen, it can catalyze the oxidation of coelenterazine to coelenteramide , and emit bioluminescence with a wavelength of about 480 nm during the oxidation process . Both bioluminescence can be measured by chemiluminescence instrument. The detection principle is shown in the figure:
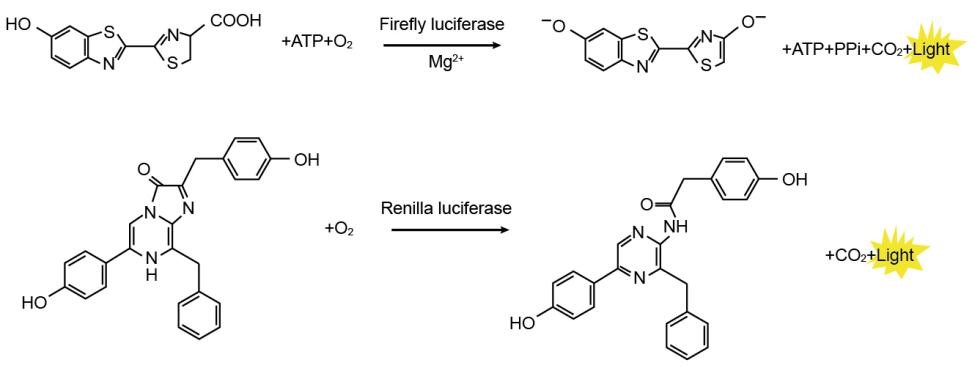
Figure 1 : Schematic diagram of firefly and Renilla luciferase assays
Usually , the 5UTR or promoter of the target gene is cloned upstream of Firefly Luciferase , or the 3UTR is cloned downstream of Firefly Luciferase , and the transcriptional regulation of the promoter or regulatory element is detected by detecting the amount of firefly luciferase. Renilla Luciferase is used as an internal reference to eliminate differences in cell number, transfection efficiency, etc. The Dual Luciferase Reporter Gene Assay Kit first uses luciferin as a substrate to detect the activity of the firefly luciferase reporter gene, and then uses coelenterazine as a substrate to detect the activity of the Renilla luciferase reporter gene while quenching the fluorescence reaction. This kit has the characteristics of high sensitivity.
Product composition
serial number | Components | Product Number / Specification | |
HRK1208 ( 100T ) | HRK1209 ( 1000T ) | ||
hrk1208-A | Cell lysis buffer | 60 mL | 60 mL × 10 |
hrk1208-B | Firefly Luciferase Buffer | 10 mL | 10 mL × 10 |
hrk1208-C | Firefly luciferase substrate ( 50 × ) | 200 μL | 200 μL × 10 |
hrk1208-D | Renilla luciferase buffer | 10 mL | 10 mL × 10 |
hrk1208 -E | Renilla luciferase substrate ( 50× ) | 200 μL | 200 μL×10 |
Transportation and storage methods
Transported by dry ice. Store at -20℃ , expiration date 1 year.
Firefly luciferase reaction working solution and Renilla luciferase reaction working solution should be prepared and used immediately, and should not be repeatedly frozen and thawed. It is recommended to store them in aliquots at -20℃ or -80℃ .
Experimental procedures
I. Pre -treatment
1. Cells
1 ) Construct the corresponding vector.
2 ) Please refer to the relevant instructions for transfection steps.
3 ) Mix the cell lysis buffer thoroughly and add the cell lysis buffer as follows to fully lyse the cells.
a: For adherent cells , aspirate the cell culture medium, add cell lysis solution according to the proportion in the table below, and gently rotate the culture dish or culture plate to completely cover the cells with lysis solution;
b: For suspended cells , centrifuge and discard the supernatant, then add lysis buffer according to the proportions in the table below.
Cell culture plates | 96- well plate | 48- well plate | 24- well plate | 12- well plate | 6- well plate |
Lysis buffer added | 100 μL | 150 μL | 200 μL | 300 μL | 500 μL |
4) Incubate for 5 min to fully lyse the cells.
[Note] : The lysate can be stored at room temperature for 6 hours , at 4°C for 16 hours , and at -80°C for a long time. (The lysate cannot be repeatedly frozen and thawed).
5 ) (Optional) Centrifuge at 10,000-16,000 rpm for 1 min and collect the supernatant .
2. Leaf tissue (taking tobacco leaves as an example, for reference only)
1) Construct the corresponding vector.
2) Pick a single colony of Agrobacterium transformed with the recombinant plasmid, inoculate it into 2 mL LB liquid medium (add the corresponding antibiotics), and culture it overnight at 28℃ 220 rpm.
3) Cultivate Agrobacterium to OD600 of 1.0, collect the cells by centrifugation at 1700× g for 5 min, and wash the cells twice with 1/2MS liquid medium; adjust the OD600 of Agrobacterium to 1.0 with 1/2MS liquid medium containing 150 μmol/L acetosyringone.
4) Mix the Agrobacterium culture solutions to be tested so that the OD600 of each culture solution is 0.5.
5) Select a fully extended tobacco leaf with a growth period of about 1 month, and inject the mixed culture solution from the back of the tobacco leaf with a 1 mL syringe (without the needle). To ensure the consistency of the experimental results, the control vector and the target vector to be tested need to be injected into different parts of the same leaf to ensure the same growth background.
6) Under normal greenhouse growth conditions, samples can be taken for observation after 24-48 hours.
7) Take 3-4 leaf discs with a diameter of 6-8 mm, put them into a 2 mL EP tube (put 3-4 small steel balls in advance), freeze them in liquid nitrogen, and grind them using a crusher (45 Hz, 30 s). After complete crushing, add 100 μL of lysis solution to the EP tube.
8) Incubate on ice for about 5 minutes to fully lyse the leaves.
9) Centrifuge at 1 minute and take the supernatant.
3. Protoplasts (for reference only)
1) Construct the corresponding vector.
2) Prepare protoplasts.
3) Add the corresponding vector to a 2 mL EP tube (the amount to be added needs to be explored), and add 100 μL of protoplast suspension. After gently shaking to mix, add 110 μL of PEG-CaCl2 solution and flick to mix. Place at room temperature for 10-15 min.
4) Add 440 μL of W5 solution and turn upside down to stop transformation.
5) Centrifuge at 200 × g for 5 min at room temperature, discard the supernatant, and add 800 μL of WI solution to resuspend the protoplasts.
6) Incubate at room temperature in the dark for 16-24 h.
7) Add the protoplasts to a 2 mL centrifuge tube, collect the protoplasts by centrifugation, and add about 100 μL of lysis solution.
8) Incubate on ice for about 5 min to fully lyse the protoplasts.
9) (Optional) Centrifuge at 10,000-16,000 rpm for 1 min and take the supernatant.
II. Fluorescence Detection
1) Take 20 μL of lysis solution and add it to the culture plate. According to the experimental needs, 3-5 wells can be set up for replication.
2) Prepare firefly luciferase reaction working solution and Renilla luciferase reaction solution, that is, dilute firefly luciferase substrate (50×) and Renilla luciferase substrate (50×)
to 1× working solution with corresponding buffers. And incubate to room temperature.
3) Add 100 μL of firefly luciferase reaction solution, shake the plate to mix, and detect the activity of firefly luciferase. The detection should be completed within 30 min.
4) Add 100 μL of Renilla luciferase reaction solution, shake the plate to mix, and detect the activity of Renilla luciferase. The detection should be completed within 30 min.
5) Analyze the data.
① Experimental design: According to different experimental purposes, a control group, an experimental group and a blank control group should be set up in each culture plate. In order to ensure the accuracy of the experiment, theoretically, the luminescence measurement values of firefly and Renilla luciferase in the blank control group should be subtracted from each experimental group (including the control group).
a. Blank control group:
Background F: untransfected cells + firefly luciferase detection reagent.
Background R: untransfected cells + firefly luciferase detection reagent + Renilla luciferase detection reagent.
Note: The sample volume of the blank control group must be the same as the experimental sample volume, containing the same culture medium/serum combination as the experimental sample, and adding exactly the same detection reagent.
b. Experimental group: transfected cells are treated with experimental compounds (i.e., experimental group F and experimental group R).
c. Control group: transfected cells are not treated to standardize the results (i.e., control group F and control group R).
② Calculation results:
Experimental group ratio = (experimental group F-background F)/(experimental group R-background R).
Control group ratio = (control group F-background F)/(control group R-background R).
Expression fold = experimental group ratio/control group ratio.

Figure 2: Flowchart of Firefly and Renilla luciferase assays in cell samples
Precautions
1 ) The consumables and equipment that need to be prepared during the test include the following: PBS ; 100 μL pipette or pipette gun; opaque white ELISA plate; Luminometer , multifunctional ELISA reader or other instruments capable of detecting bioluminescence;
2 ) Reaction temperature: Enzymatic reactions are sensitive to temperature. All reagents must be equilibrated to room temperature ( 20-25°C ) before use;
3 ) Detection instrument: Any instrument that can detect chemiluminescence is applicable, but due to different settings and sensitivities of different instruments, the measured light signal values will also be different;

